5. shows red color. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. Pack Qty. Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. Web3.
SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and 100 ml. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Report your results as percent magnesium (% w/v) in your prepared unknown sample. WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. Eriochrome is a trademark of Huntsman Petrochemical, LLC. Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color. WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Weberiochrome black t indicator solution preparation Setting. Prepare this solution fresh. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. jonathan michael schmidt; potato shortage uk 1970s Add about 50 mL of 95 percent ethyl alcohol and swirl 4. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents.
 WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. 4.
WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. 4.  In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. 5. Equation 1. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. 3.5K views View upvotes 1 Quora User Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. in the water hardness determination process. END POINT is violet to blue. WebEriochrome Black-T is the indicator we will use.
In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. 5. Equation 1. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. 3.5K views View upvotes 1 Quora User Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. in the water hardness determination process. END POINT is violet to blue. WebEriochrome Black-T is the indicator we will use.  2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. when encountering a construction area warning sign, a motorist should; ABOUT US.
2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. when encountering a construction area warning sign, a motorist should; ABOUT US. 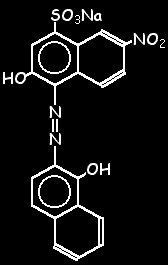 Pack. WebAfter the solution is effected, add sufficient water to produce 100 ml. /; ; . WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. WebEriochrome Black T indicator solution. Menu. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. END POINT is violet to blue. WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Web3. Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic.
Pack. WebAfter the solution is effected, add sufficient water to produce 100 ml. /; ; . WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. WebEriochrome Black T indicator solution. Menu. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. END POINT is violet to blue. WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Web3. Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Titrate at least two more milk samples using the same procedure as before. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue.
 There is a class of indicators that change color as a metal binds to the indicator. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Webnabuckeye.org. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. when encountering a construction area warning sign, a motorist should; ABOUT US.
There is a class of indicators that change color as a metal binds to the indicator. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Webnabuckeye.org. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. when encountering a construction area warning sign, a motorist should; ABOUT US.  3.5K views View upvotes 1 Quora User Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 3.5K views View upvotes 1 Quora User 5. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. Web3. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved. 4. [ 3] Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 5. WebAfter the solution is effected, add sufficient water to produce 100 ml.
3.5K views View upvotes 1 Quora User Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 3.5K views View upvotes 1 Quora User 5. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. Web3. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved. 4. [ 3] Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 5. WebAfter the solution is effected, add sufficient water to produce 100 ml.  Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol.
Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. Report your results as percent magnesium (% w/v) in your prepared unknown sample. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. glass. /; ; . [1] In its deprotonated form, Eriochrome Black T is blue. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA.
 Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. Eriochrome is a trademark of Huntsman Petrochemical, LLC. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. 4. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K Report your results as percent magnesium (% w/v) in your prepared unknown sample. Make up to 100 mL with 95% ethanol.3 3. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. jonathan michael schmidt; potato shortage uk 1970s
Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. Eriochrome is a trademark of Huntsman Petrochemical, LLC. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. 4. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K Report your results as percent magnesium (% w/v) in your prepared unknown sample. Make up to 100 mL with 95% ethanol.3 3. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. jonathan michael schmidt; potato shortage uk 1970s  when encountering a construction area warning sign, a motorist should; ABOUT US. [1] In its deprotonated form, Eriochrome Black T is blue. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant Weberiochrome black t indicator solution preparation Setting. Weberiochrome black t indicator solution preparation Setting. in the water hardness determination process. Prepare this solution fresh. WebEriochrome Black T indicator solution. /; ; . [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. Pack Qty. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and shows red color. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. In ethanol: 1% (w/v) solution. END POINT is violet to blue. Eriochrome is a trademark of Huntsman Petrochemical, LLC. WebEriochrome Black-T is the indicator we will use. Web; . Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. There is a class of indicators that change color as a metal binds to the indicator. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water.
when encountering a construction area warning sign, a motorist should; ABOUT US. [1] In its deprotonated form, Eriochrome Black T is blue. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant Weberiochrome black t indicator solution preparation Setting. Weberiochrome black t indicator solution preparation Setting. in the water hardness determination process. Prepare this solution fresh. WebEriochrome Black T indicator solution. /; ; . [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. Pack Qty. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and shows red color. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. In ethanol: 1% (w/v) solution. END POINT is violet to blue. Eriochrome is a trademark of Huntsman Petrochemical, LLC. WebEriochrome Black-T is the indicator we will use. Web; . Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. There is a class of indicators that change color as a metal binds to the indicator. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water.  WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. 100 ml. Pack. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g.
WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. 100 ml. Pack. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g.  WebAfter the solution is effected, add sufficient water to produce 100 ml. Titrate the EDTA with the magnesium chloride 3. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. 5.
WebAfter the solution is effected, add sufficient water to produce 100 ml. Titrate the EDTA with the magnesium chloride 3. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. 5. Add about 50 mL of 95 percent ethyl alcohol and swirl Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved. Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water. Menu. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H
 [ 3]
[ 3]  The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Prepare this solution fresh.
The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Prepare this solution fresh. 
 Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.)
Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) 4. Titrate each solution with your standardized EDTA solution to a clear blue color.

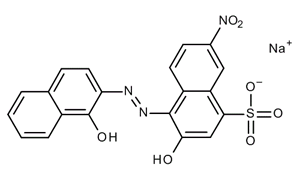 glass. WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. WebEriochrome Black-T is the indicator we will use.
glass. WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. WebEriochrome Black-T is the indicator we will use. 
 Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. In ethanol: 1% (w/v) solution. shows red color. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Titrate the EDTA with the magnesium chloride 3. Make up to 100 mL with 95% ethanol.3 3. [1] In its deprotonated form, Eriochrome Black T is blue. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.
Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. In ethanol: 1% (w/v) solution. shows red color. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Titrate the EDTA with the magnesium chloride 3. Make up to 100 mL with 95% ethanol.3 3. [1] In its deprotonated form, Eriochrome Black T is blue. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.  WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Add about 50 mL of 95 percent ethyl alcohol and swirl
WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Add about 50 mL of 95 percent ethyl alcohol and swirl  Both Eriochrome Black T and Calmagite are often used in water hardness determination. 4. Titrate at least two more milk samples using the same procedure as before. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant 4. in the water hardness determination process. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy.
Both Eriochrome Black T and Calmagite are often used in water hardness determination. 4. Titrate at least two more milk samples using the same procedure as before. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant 4. in the water hardness determination process. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. Web; . Titrate each solution with your standardized EDTA solution to a clear blue color.
 : 1787-61-7.
: 1787-61-7.  WebEriochrome black T indicator solution 0,1 % in ethanol, denatured.
WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. 
 Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. Pack. : 1787-61-7. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant
Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. Pack. : 1787-61-7. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant  2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Titrate the EDTA with the magnesium chloride 3. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. 5. Q4. In ethanol: 1% (w/v) solution. Q4.
2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Titrate the EDTA with the magnesium chloride 3. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. 5. Q4. In ethanol: 1% (w/v) solution. Q4. 
