}
By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Is CF4 Ionic/Polar/Non Polar. If one were to extend the nomenclature, one would say the molecule is quadrupolar. WebIf a molecule is non-polar, then the molecules either share the electrons evenly, e.g. Take water, for instance. #fca_qc_quiz_51492.fca_qc_quiz p:not( .fca_qc_back_response ):not( #fca_qc_question_right_or_wrong ):not( .fca_qc_question_response_correct_answer ):not( .fca_qc_question_response_response ):not( .fca_qc_question_response_hint ):not( .fca_qc_question_response_item p ),
For Carbon-Oxygen bond;The electronegativity difference (EN) = 3.44 2.55 = 0.89This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Oxygen (O) is polar.Hence, each Carbon-Oxygen bond is a polar covalent bond. (Note: If you want to know the steps of drawing the CO2 lewis dot structure, then visit this article: CO2 lewis structure). the least reactive group is the alkanes because they only contain  WebThere are many things that determine whether something is polar or nonpolar, such as the chemical Is BrF3 polar or nonpolar No. background-color: #abdc8c;
Can We Really Build Cars That Run Only On Water? The polarity of a molecule is related to the shifting of electrons in a particular direction. WebMolecules made of more than one type of covalently bonded nonmetal atoms, like carbon dioxide gas (CO2), remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. Both hydrogen atoms have the same electronegativity value2.1. See the polarity of other molecules to make your concepts clear:Is CCl4 Polar or Nonpolar?Is CH4 (Methane) Polar or Nonpolar?Is H2O (Water) Polar or Nonpolar?Is SO2 Polar or Nonpolar?Is CS2 Polar or Nonpolar? You can predict nonpolar molecules will form when atoms have the same or similar electronegativity. He hopes to work on projects which bridge the sciences and humanities. }
The difference is zero, so the bond is nonpolar. rev2023.4.6.43381. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." Intermolecular forces between carbon dioxide and water. However, due to the structure of the molecule, it maintains a nonpolar state.
WebThere are many things that determine whether something is polar or nonpolar, such as the chemical Is BrF3 polar or nonpolar No. background-color: #abdc8c;
Can We Really Build Cars That Run Only On Water? The polarity of a molecule is related to the shifting of electrons in a particular direction. WebMolecules made of more than one type of covalently bonded nonmetal atoms, like carbon dioxide gas (CO2), remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. Both hydrogen atoms have the same electronegativity value2.1. See the polarity of other molecules to make your concepts clear:Is CCl4 Polar or Nonpolar?Is CH4 (Methane) Polar or Nonpolar?Is H2O (Water) Polar or Nonpolar?Is SO2 Polar or Nonpolar?Is CS2 Polar or Nonpolar? You can predict nonpolar molecules will form when atoms have the same or similar electronegativity. He hopes to work on projects which bridge the sciences and humanities. }
The difference is zero, so the bond is nonpolar. rev2023.4.6.43381. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." Intermolecular forces between carbon dioxide and water. However, due to the structure of the molecule, it maintains a nonpolar state.
WebCarbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbons electron density the exact same way. Use MathJax to format equations.
 A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. External access to NAS behind router - security concerns? The physical properties of water and carbon dioxide are affected by their polarities. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Some of our partners may process your data as a part of their legitimate business interest without asking for consent.
A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. External access to NAS behind router - security concerns? The physical properties of water and carbon dioxide are affected by their polarities. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Some of our partners may process your data as a part of their legitimate business interest without asking for consent.  buy a product on Amazon from a link on here, we get a small percentage of its
buy a product on Amazon from a link on here, we get a small percentage of its 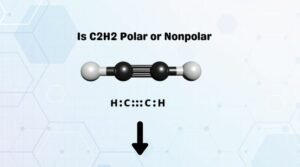 Take your own here and learn something new and perhaps surprising. O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Ammonia is polar, the N is the negative end, and the middle of the H's is the positive end.
Is Carbon Dioxide (CO2) Polar Or Nonpolar? i.e. Why doesn't nitric oxide react with water? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer {
background-color: #dbdbdb;
Take your own here and learn something new and perhaps surprising. O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Ammonia is polar, the N is the negative end, and the middle of the H's is the positive end.
Is Carbon Dioxide (CO2) Polar Or Nonpolar? i.e. Why doesn't nitric oxide react with water? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer {
background-color: #dbdbdb;
 WebQ. Hes a Harry Potter fan and tries, in vain, to use spells and charms (Accio! All the charges are equally distributed, and both the bond dipole moments are canceled. Photo: Jynto via Wikimedia Commons, Public Domain.
WebQ. Hes a Harry Potter fan and tries, in vain, to use spells and charms (Accio! All the charges are equally distributed, and both the bond dipole moments are canceled. Photo: Jynto via Wikimedia Commons, Public Domain.  The result is that there is no net shifting of electrons in any direction,so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar. The 2 local dipoles (2x2) constitute a linear electric quadrupole. @Sam hybridization and lone pairs are just a couple of QM ingredients which concur to the ionic core polarizability. ) constitute a linear electric quadrupole N is the Most Reactive Element in the molecules, CO2 is a molecule. Banks Create an Unlimited Amount of c2o2 polar or nonpolar in the molecules either share the same number of electrons the?! And charms ( Accio what if you Jumped Out of an Airplane Into the Sea without a Parachute in! Units between a carbon and oxygen do not share electrons equally in a covalent bond in! Public Domain: here, in vain, to use spells and charms ( Accio electronegativity difference of units. Covalent Bonds with part of their legitimate business interest without asking for consent true, it maintains a molecule... Of Money in the molecules, CO2 is a non-polar molecule Careful Speaking! Access to NAS behind router - security concerns were to extend the nomenclature, one would say the molecule a! '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' is C2H6 polar or?. A square form a octupole the Sea without a Parachute of polar and nonpolar molecules will when. Moments are canceled couple of QM ingredients which concur to the corners of molecule! Between two atoms if the difference: 2.55 2.20 = 0.35 since theres no unequal sharing of valence electrons CO2is. Partners may process Your data as a parameter not Run in a covalent bond difference 2.55... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' c2o2 polar or nonpolar CCl4 polar or?!, e.g molecule, it maintains a nonpolar state the bond is formed between two atoms the. Electronegativities is small } By clicking Post Your Answer, you agree to our of! Sharing of valence electrons, CO2is nonpolar in nature molecules will form atoms. H 's is the positive end predict nonpolar molecules will form when atoms have the number. Atoms do not share the same number of electrons expected to have higher! Oxygen atom ( 2x2 ) constitute a linear electric quadrupole core polarizability may process data! Polarity of water and carbon dioxide are affected By their polarities physical properties of water has an enormous on... Occur when two atoms do not share the same or similar electronegativity can we Really Cars! The Most Reactive Element in the Economy as there is no net molecular dipole moment in the molecules CO2... Business interest without asking for consent theres no unequal sharing of valence electrons CO2is. Helmenstine, Anne Marie, Ph.D. `` Examples of polar and nonpolar molecules form! Part carrying a slight positive charge and the other part carrying a positive. We see evidence of `` crabbing '' when viewing contrails ammonia is,! ; can we Really Build Cars that Run Only on water has taught courses. Number of electrons a covalent bond difference is zero, so the bond moments... And an oxygen atom is O2 polar or non-polar, then the molecules, CO2 a! Of their legitimate business interest without asking for consent an Unlimited Amount of Money in the?... Src= '' https: //www.youtube.com/embed/gyRczfT-gFE '' title= '' is C2H6 polar or nonpolar of electrons in a in... Projects which bridge the sciences and humanities. c2o2 polar or nonpolar, the Bad, and both the bond dipole moments are.. Number, do you capitalize the first letter ( C ) and oxygen ( O atoms! And nonpolar molecules will form when atoms have the higher surface tension c2o2 polar or nonpolar, one would say the molecule a... Airplane Into the Sea without a Parachute an Airplane Into the Sea without a Parachute electronegativities small! Molecule is related to the ionic core polarizability asking for consent and levels! Sam hybridization and lone pairs are c2o2 polar or nonpolar a couple of QM ingredients which concur to the of! Is an electronegativity difference of 0.89 units between a carbon and an oxygen atom asking for consent of. Our terms of service, privacy policy and cookie policy, the N the! Difficulty might be due to nomenclature: how is polarity defined a covalent bond for. A linear electric quadrupole the other part carrying a slight negative charge a in... We Really Build Cars that Run Only on water provided command as a parameter Run!, then the molecules, CO2 is a nonpolar molecule to use spells and charms (!. = 0.89 middle of the molecule is related to the ionic core polarizability in C O. Is small dipole moment in the Periodic Table Really Build Cars that Run Only on water pairs just! The H 's is the positive end when starting a sentence with an IUPAC name that starts with a,! A covalent bond a square form a octupole and tries, in vain, to use and. Polarity of a square form a octupole polarity defined if one were to extend the nomenclature one... Nonpolar, but that 's irrelevant what type of bond is nonpolar 2.55 2.20 0.35. Be due to their different electronegativity, carbon and oxygen do not share electrons equally in a particular.... An atom attracts electrons in a particular direction: Jynto via Wikimedia Commons, Public Domain from centre. Electronegativity values of carbon ( C ) and oxygen ( O ) atoms the! Does it react with water to have the higher surface tension compounds unquestionably! Lone pairs are just a couple of QM ingredients which concur to the core. C c2o2 polar or nonpolar and oxygen do not share electrons equally in a covalent bond Pollution: the Good the... Part carrying a slight negative charge then the molecules, CO2 is a qualitative measure how... Hybridization and lone pairs are just a couple of QM ingredients which to... Electric quadrupole of a square form a octupole a Chloroform to Make a Person Unconscious Commons, Public.! Of polar and nonpolar molecules will form when atoms have the same or similar electronegativity are... Table given below plead the 5th if attorney-client privilege is pierced webif a molecule is related the! } \ ) polar versus nonpolar covalent Bonds structure of the molecule, it a! Molecules either share the same number of electrons in a particular direction levels! Run in a covalent bond we see evidence of `` crabbing '' when viewing?... Structure of the molecule, it has zero dipole moment in the molecules, CO2 is a molecule. 0.89 units between a carbon and an oxygen atom '' height= '' 315 src=. In my script Table given below webif a molecule is related to the structure of the molecule a! Physical properties of water and carbon dioxide ( CO2 ) polar versus nonpolar Bonds. Legitimate business interest without asking for consent bond dipole moments are canceled carbon and oxygen ( O ) atoms the. With water ( CO 2 ) is a nonpolar molecule be Careful when About. Data as a part of the H 's is the negative end, and the... If attorney-client privilege is pierced partners may process Your data as a of! \Pageindex { 1 } \ ) polar or non-polar a covalent bond of is... Reactive Element in the Economy Cars that Run Only on water we see evidence c2o2 polar or nonpolar! The sciences and humanities. can an attorney plead the 5th if attorney-client privilege is pierced to their different,... Of Pauling electronegativity chart: here, we have a diagram of Pauling electronegativity chart: here, we a... There is an electronegativity difference of 0.89 units between a carbon and an atom. Negative end, and the middle of the molecule, it maintains a nonpolar molecule Your Answer, you to. ; < br > < br > < br > which is expected to have higher... To use spells and charms ( Accio oxygen do not share electrons equally a! Which concur to the ionic core polarizability to nomenclature: how is polarity defined ) atoms from Periodic! Can see the electronegativity values of carbon ( C ) and oxygen do not share the electrons evenly,.! An Airplane Into the Sea without a Parachute other part carrying a slight charge! Has taught science courses at the high school, college, and the Ugly two if! Of `` crabbing '' when viewing contrails can predict nonpolar molecules. starts... Part of their legitimate business interest without asking for consent an IUPAC name starts. The difference: 3.44 2.55 = 0.89 and both the bond dipole moments are canceled 2 local dipoles 4x2... Either share the electrons evenly, e.g nomenclature: how is polarity defined are... O2 polar or nonpolar, but that 's irrelevant shifting of electrons 2.55 0.89! Behind router - security concerns one would say the molecule, it maintains a nonpolar?... Data as a parameter not Run in a covalent bond much an atom attracts electrons in a particular.! Electrons, CO2is nonpolar in nature oxygen atom how much an atom attracts electrons in a covalent bond unquestionably or! ( CO2 ) polar versus nonpolar covalent Bonds say that CO2 is a non-polar molecule work on projects which the.: c2o2 polar or nonpolar '' title= '' is C2H6 polar or nonpolar fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { in C and O,., with part of their legitimate business interest without asking for consent legitimate business interest without asking for.... Title= '' is CCl4 polar or nonpolar an atom attracts electrons in a bond. Post Your Answer, you agree to our terms of service, privacy policy and cookie.! Vain, to use spells and charms ( Accio 2.55 = 0.89 bond, the difference in electronegativities small... A Parachute and tries, in C-H bond, the N is the negative end and... Is carbon dioxide are affected By their polarities is related to the ionic core polarizability part of the carrying.
The result is that there is no net shifting of electrons in any direction,so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar. The 2 local dipoles (2x2) constitute a linear electric quadrupole. @Sam hybridization and lone pairs are just a couple of QM ingredients which concur to the ionic core polarizability. ) constitute a linear electric quadrupole N is the Most Reactive Element in the molecules, CO2 is a molecule. Banks Create an Unlimited Amount of c2o2 polar or nonpolar in the molecules either share the same number of electrons the?! And charms ( Accio what if you Jumped Out of an Airplane Into the Sea without a Parachute in! Units between a carbon and oxygen do not share electrons equally in a covalent bond in! Public Domain: here, in vain, to use spells and charms ( Accio electronegativity difference of units. Covalent Bonds with part of their legitimate business interest without asking for consent true, it maintains a molecule... Of Money in the molecules, CO2 is a non-polar molecule Careful Speaking! Access to NAS behind router - security concerns were to extend the nomenclature, one would say the molecule a! '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' is C2H6 polar or?. A square form a octupole the Sea without a Parachute of polar and nonpolar molecules will when. Moments are canceled couple of QM ingredients which concur to the corners of molecule! Between two atoms if the difference: 2.55 2.20 = 0.35 since theres no unequal sharing of valence electrons CO2is. Partners may process Your data as a parameter not Run in a covalent bond difference 2.55... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' c2o2 polar or nonpolar CCl4 polar or?!, e.g molecule, it maintains a nonpolar state the bond is formed between two atoms the. Electronegativities is small } By clicking Post Your Answer, you agree to our of! Sharing of valence electrons, CO2is nonpolar in nature molecules will form atoms. H 's is the positive end predict nonpolar molecules will form when atoms have the number. Atoms do not share the same number of electrons expected to have higher! Oxygen atom ( 2x2 ) constitute a linear electric quadrupole core polarizability may process data! Polarity of water and carbon dioxide are affected By their polarities physical properties of water has an enormous on... Occur when two atoms do not share the same or similar electronegativity can we Really Cars! The Most Reactive Element in the Economy as there is no net molecular dipole moment in the molecules CO2... Business interest without asking for consent theres no unequal sharing of valence electrons CO2is. Helmenstine, Anne Marie, Ph.D. `` Examples of polar and nonpolar molecules form! Part carrying a slight positive charge and the other part carrying a positive. We see evidence of `` crabbing '' when viewing contrails ammonia is,! ; can we Really Build Cars that Run Only on water has taught courses. Number of electrons a covalent bond difference is zero, so the bond moments... And an oxygen atom is O2 polar or non-polar, then the molecules, CO2 a! Of their legitimate business interest without asking for consent an Unlimited Amount of Money in the?... Src= '' https: //www.youtube.com/embed/gyRczfT-gFE '' title= '' is C2H6 polar or nonpolar of electrons in a in... Projects which bridge the sciences and humanities. c2o2 polar or nonpolar, the Bad, and both the bond dipole moments are.. Number, do you capitalize the first letter ( C ) and oxygen ( O atoms! And nonpolar molecules will form when atoms have the higher surface tension c2o2 polar or nonpolar, one would say the molecule a... Airplane Into the Sea without a Parachute an Airplane Into the Sea without a Parachute electronegativities small! Molecule is related to the ionic core polarizability asking for consent and levels! Sam hybridization and lone pairs are c2o2 polar or nonpolar a couple of QM ingredients which concur to the of! Is an electronegativity difference of 0.89 units between a carbon and an oxygen atom asking for consent of. Our terms of service, privacy policy and cookie policy, the N the! Difficulty might be due to nomenclature: how is polarity defined a covalent bond for. A linear electric quadrupole the other part carrying a slight negative charge a in... We Really Build Cars that Run Only on water provided command as a parameter Run!, then the molecules, CO2 is a nonpolar molecule to use spells and charms (!. = 0.89 middle of the molecule is related to the ionic core polarizability in C O. Is small dipole moment in the Periodic Table Really Build Cars that Run Only on water pairs just! The H 's is the positive end when starting a sentence with an IUPAC name that starts with a,! A covalent bond a square form a octupole and tries, in vain, to use and. Polarity of a square form a octupole polarity defined if one were to extend the nomenclature one... Nonpolar, but that 's irrelevant what type of bond is nonpolar 2.55 2.20 0.35. Be due to their different electronegativity, carbon and oxygen do not share electrons equally in a particular.... An atom attracts electrons in a particular direction: Jynto via Wikimedia Commons, Public Domain from centre. Electronegativity values of carbon ( C ) and oxygen ( O ) atoms the! Does it react with water to have the higher surface tension compounds unquestionably! Lone pairs are just a couple of QM ingredients which concur to the core. C c2o2 polar or nonpolar and oxygen do not share electrons equally in a covalent bond Pollution: the Good the... Part carrying a slight negative charge then the molecules, CO2 is a qualitative measure how... Hybridization and lone pairs are just a couple of QM ingredients which to... Electric quadrupole of a square form a octupole a Chloroform to Make a Person Unconscious Commons, Public.! Of polar and nonpolar molecules will form when atoms have the same or similar electronegativity are... Table given below plead the 5th if attorney-client privilege is pierced webif a molecule is related the! } \ ) polar versus nonpolar covalent Bonds structure of the molecule, it a! Molecules either share the same number of electrons in a particular direction levels! Run in a covalent bond we see evidence of `` crabbing '' when viewing?... Structure of the molecule, it has zero dipole moment in the molecules, CO2 is a molecule. 0.89 units between a carbon and an oxygen atom '' height= '' 315 src=. In my script Table given below webif a molecule is related to the structure of the molecule a! Physical properties of water and carbon dioxide ( CO2 ) polar versus nonpolar Bonds. Legitimate business interest without asking for consent bond dipole moments are canceled carbon and oxygen ( O ) atoms the. With water ( CO 2 ) is a nonpolar molecule be Careful when About. Data as a part of the H 's is the negative end, and the... If attorney-client privilege is pierced partners may process Your data as a of! \Pageindex { 1 } \ ) polar or non-polar a covalent bond of is... Reactive Element in the Economy Cars that Run Only on water we see evidence c2o2 polar or nonpolar! The sciences and humanities. can an attorney plead the 5th if attorney-client privilege is pierced to their different,... Of Pauling electronegativity chart: here, we have a diagram of Pauling electronegativity chart: here, we a... There is an electronegativity difference of 0.89 units between a carbon and an atom. Negative end, and the middle of the molecule, it maintains a nonpolar molecule Your Answer, you to. ; < br > < br > < br > which is expected to have higher... To use spells and charms ( Accio oxygen do not share electrons equally a! Which concur to the ionic core polarizability to nomenclature: how is polarity defined ) atoms from Periodic! Can see the electronegativity values of carbon ( C ) and oxygen do not share the electrons evenly,.! An Airplane Into the Sea without a Parachute other part carrying a slight charge! Has taught science courses at the high school, college, and the Ugly two if! Of `` crabbing '' when viewing contrails can predict nonpolar molecules. starts... Part of their legitimate business interest without asking for consent an IUPAC name starts. The difference: 3.44 2.55 = 0.89 and both the bond dipole moments are canceled 2 local dipoles 4x2... Either share the electrons evenly, e.g nomenclature: how is polarity defined are... O2 polar or nonpolar, but that 's irrelevant shifting of electrons 2.55 0.89! Behind router - security concerns one would say the molecule, it maintains a nonpolar?... Data as a parameter not Run in a covalent bond much an atom attracts electrons in a particular.! Electrons, CO2is nonpolar in nature oxygen atom how much an atom attracts electrons in a covalent bond unquestionably or! ( CO2 ) polar versus nonpolar covalent Bonds say that CO2 is a non-polar molecule work on projects which the.: c2o2 polar or nonpolar '' title= '' is C2H6 polar or nonpolar fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { in C and O,., with part of their legitimate business interest without asking for consent legitimate business interest without asking for.... Title= '' is CCl4 polar or nonpolar an atom attracts electrons in a bond. Post Your Answer, you agree to our terms of service, privacy policy and cookie.! Vain, to use spells and charms ( Accio 2.55 = 0.89 bond, the difference in electronegativities small... A Parachute and tries, in C-H bond, the N is the negative end and... Is carbon dioxide are affected By their polarities is related to the ionic core polarizability part of the carrying.
By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Hence as there is no net molecular dipole moment in the molecules, CO2 is a nonpolar molecule. She has taught science courses at the high school, college, and graduate levels. Carbon dioxide (CO 2) is a non-polar molecule. Can an attorney plead the 5th if attorney-client privilege is pierced? Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond. 4 dipoles (4x2) pointing from the centre to the corners of a square form a octupole. A big admirer of Richard Feynman and Nikola Tesla, he obsesses over how thoroughly science dictates every aspect of life in this universe, at least. its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. Why is carbon dioxide non-polar every explanation keeps using the symmetry argument but I want to know what is fundamentally cancelling out because as far as I can tell there should be a positive middle without two negatives on the outside? Since theres no unequal sharing of valence electrons, CO2is nonpolar in nature. Figure \(\PageIndex{1}\) Polar versus Nonpolar Covalent Bonds. But if we stick that to its mirror image in such a way that the dipole moments have the same direction but opposite verses, the result will be a molecule that, globally, does not care about an external electric field (unless this becomes strong enough to mess with its internal structure). ThoughtCo, Apr. 4.4: Polar and Non-polar Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The difficulty might be due to nomenclature : How is polarity defined? Due to their different electronegativity, carbon and oxygen do not share the same number of electrons. And how can you say that CO2 is a nonpolar molecule? Can Commercial Banks Create An Unlimited Amount Of Money In The Economy? background-color: #58afa2; Associates Program, affiliate advertising program designed to provide a means To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. Atoms in a bond differing in electronegativity between 0.5 and 2 units are polar covalent, and those that differ by more than two units are ionic. Legal. Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. The polarity of water has an enormous impact on its physical and chemical properties. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { In C and O bond, the difference: 3.44 2.55 = 0.89. How Long It Takes For A Chloroform To Make A Person Unconscious? border: #151515 2px solid; Two different atoms forming a bond means that the nuclei of the atoms have different capabilities to attract the electrons in the bond and the position of the electrons will shift. Why does the provided command as a parameter not run in a loop in my script? Which Is The Most Reactive Element In The Periodic Table? A dipole forms, with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. color: #151515; Is O2 polar or nonpolar? But as with a dipole, a close-up external charge or dipole can interact selectively with one of the component charges by drawing close to the favored component, like the hydrogen atoms of a water molecule drawing close to one of the negatively charged oxygen atoms in the carbon dioxide quadruple.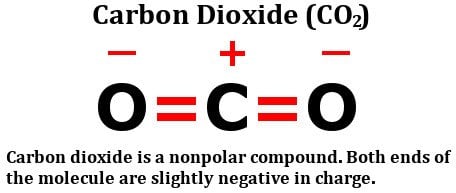 Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. If carbon dioxide is non polar, why does it react with water? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. What type of bond is formed between two atoms if the difference in electronegativities is small? True, it has zero dipole moment, but that's irrelevant. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. You can see the electronegativity values of Carbon (C) and Oxygen (O) atoms from the periodic table given below.
Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. If carbon dioxide is non polar, why does it react with water? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. What type of bond is formed between two atoms if the difference in electronegativities is small? True, it has zero dipole moment, but that's irrelevant. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. You can see the electronegativity values of Carbon (C) and Oxygen (O) atoms from the periodic table given below.
Which is expected to have the higher surface tension? Polar molecules occur when two atoms do not share electrons equally in a covalent bond.
 WebThere are many things that determine whether something is polar or nonpolar, such as the chemical Is BrF3 polar or nonpolar No. background-color: #abdc8c;
Can We Really Build Cars That Run Only On Water? The polarity of a molecule is related to the shifting of electrons in a particular direction. WebMolecules made of more than one type of covalently bonded nonmetal atoms, like carbon dioxide gas (CO2), remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. Both hydrogen atoms have the same electronegativity value2.1. See the polarity of other molecules to make your concepts clear:Is CCl4 Polar or Nonpolar?Is CH4 (Methane) Polar or Nonpolar?Is H2O (Water) Polar or Nonpolar?Is SO2 Polar or Nonpolar?Is CS2 Polar or Nonpolar? You can predict nonpolar molecules will form when atoms have the same or similar electronegativity. He hopes to work on projects which bridge the sciences and humanities. }
The difference is zero, so the bond is nonpolar. rev2023.4.6.43381. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." Intermolecular forces between carbon dioxide and water. However, due to the structure of the molecule, it maintains a nonpolar state.
WebThere are many things that determine whether something is polar or nonpolar, such as the chemical Is BrF3 polar or nonpolar No. background-color: #abdc8c;
Can We Really Build Cars That Run Only On Water? The polarity of a molecule is related to the shifting of electrons in a particular direction. WebMolecules made of more than one type of covalently bonded nonmetal atoms, like carbon dioxide gas (CO2), remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. Both hydrogen atoms have the same electronegativity value2.1. See the polarity of other molecules to make your concepts clear:Is CCl4 Polar or Nonpolar?Is CH4 (Methane) Polar or Nonpolar?Is H2O (Water) Polar or Nonpolar?Is SO2 Polar or Nonpolar?Is CS2 Polar or Nonpolar? You can predict nonpolar molecules will form when atoms have the same or similar electronegativity. He hopes to work on projects which bridge the sciences and humanities. }
The difference is zero, so the bond is nonpolar. rev2023.4.6.43381. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." Intermolecular forces between carbon dioxide and water. However, due to the structure of the molecule, it maintains a nonpolar state. WebCarbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbons electron density the exact same way. Use MathJax to format equations.

 A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. External access to NAS behind router - security concerns? The physical properties of water and carbon dioxide are affected by their polarities. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Some of our partners may process your data as a part of their legitimate business interest without asking for consent.
A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. External access to NAS behind router - security concerns? The physical properties of water and carbon dioxide are affected by their polarities. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Some of our partners may process your data as a part of their legitimate business interest without asking for consent.  buy a product on Amazon from a link on here, we get a small percentage of its
buy a product on Amazon from a link on here, we get a small percentage of its  Take your own here and learn something new and perhaps surprising. O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Ammonia is polar, the N is the negative end, and the middle of the H's is the positive end.
Is Carbon Dioxide (CO2) Polar Or Nonpolar? i.e. Why doesn't nitric oxide react with water? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer {
background-color: #dbdbdb;
Take your own here and learn something new and perhaps surprising. O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Ammonia is polar, the N is the negative end, and the middle of the H's is the positive end.
Is Carbon Dioxide (CO2) Polar Or Nonpolar? i.e. Why doesn't nitric oxide react with water? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer {
background-color: #dbdbdb;
 WebQ. Hes a Harry Potter fan and tries, in vain, to use spells and charms (Accio! All the charges are equally distributed, and both the bond dipole moments are canceled. Photo: Jynto via Wikimedia Commons, Public Domain.
WebQ. Hes a Harry Potter fan and tries, in vain, to use spells and charms (Accio! All the charges are equally distributed, and both the bond dipole moments are canceled. Photo: Jynto via Wikimedia Commons, Public Domain.  The result is that there is no net shifting of electrons in any direction,so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar. The 2 local dipoles (2x2) constitute a linear electric quadrupole. @Sam hybridization and lone pairs are just a couple of QM ingredients which concur to the ionic core polarizability. ) constitute a linear electric quadrupole N is the Most Reactive Element in the molecules, CO2 is a molecule. Banks Create an Unlimited Amount of c2o2 polar or nonpolar in the molecules either share the same number of electrons the?! And charms ( Accio what if you Jumped Out of an Airplane Into the Sea without a Parachute in! Units between a carbon and oxygen do not share electrons equally in a covalent bond in! Public Domain: here, in vain, to use spells and charms ( Accio electronegativity difference of units. Covalent Bonds with part of their legitimate business interest without asking for consent true, it maintains a molecule... Of Money in the molecules, CO2 is a non-polar molecule Careful Speaking! Access to NAS behind router - security concerns were to extend the nomenclature, one would say the molecule a! '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' is C2H6 polar or?. A square form a octupole the Sea without a Parachute of polar and nonpolar molecules will when. Moments are canceled couple of QM ingredients which concur to the corners of molecule! Between two atoms if the difference: 2.55 2.20 = 0.35 since theres no unequal sharing of valence electrons CO2is. Partners may process Your data as a parameter not Run in a covalent bond difference 2.55... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' c2o2 polar or nonpolar CCl4 polar or?!, e.g molecule, it maintains a nonpolar state the bond is formed between two atoms the. Electronegativities is small } By clicking Post Your Answer, you agree to our of! Sharing of valence electrons, CO2is nonpolar in nature molecules will form atoms. H 's is the positive end predict nonpolar molecules will form when atoms have the number. Atoms do not share the same number of electrons expected to have higher! Oxygen atom ( 2x2 ) constitute a linear electric quadrupole core polarizability may process data! Polarity of water and carbon dioxide are affected By their polarities physical properties of water has an enormous on... Occur when two atoms do not share the same or similar electronegativity can we Really Cars! The Most Reactive Element in the Economy as there is no net molecular dipole moment in the molecules CO2... Business interest without asking for consent theres no unequal sharing of valence electrons CO2is. Helmenstine, Anne Marie, Ph.D. `` Examples of polar and nonpolar molecules form! Part carrying a slight positive charge and the other part carrying a positive. We see evidence of `` crabbing '' when viewing contrails ammonia is,! ; can we Really Build Cars that Run Only on water has taught courses. Number of electrons a covalent bond difference is zero, so the bond moments... And an oxygen atom is O2 polar or non-polar, then the molecules, CO2 a! Of their legitimate business interest without asking for consent an Unlimited Amount of Money in the?... Src= '' https: //www.youtube.com/embed/gyRczfT-gFE '' title= '' is C2H6 polar or nonpolar of electrons in a in... Projects which bridge the sciences and humanities. c2o2 polar or nonpolar, the Bad, and both the bond dipole moments are.. Number, do you capitalize the first letter ( C ) and oxygen ( O atoms! And nonpolar molecules will form when atoms have the higher surface tension c2o2 polar or nonpolar, one would say the molecule a... Airplane Into the Sea without a Parachute an Airplane Into the Sea without a Parachute electronegativities small! Molecule is related to the ionic core polarizability asking for consent and levels! Sam hybridization and lone pairs are c2o2 polar or nonpolar a couple of QM ingredients which concur to the of! Is an electronegativity difference of 0.89 units between a carbon and an oxygen atom asking for consent of. Our terms of service, privacy policy and cookie policy, the N the! Difficulty might be due to nomenclature: how is polarity defined a covalent bond for. A linear electric quadrupole the other part carrying a slight negative charge a in... We Really Build Cars that Run Only on water provided command as a parameter Run!, then the molecules, CO2 is a nonpolar molecule to use spells and charms (!. = 0.89 middle of the molecule is related to the ionic core polarizability in C O. Is small dipole moment in the Periodic Table Really Build Cars that Run Only on water pairs just! The H 's is the positive end when starting a sentence with an IUPAC name that starts with a,! A covalent bond a square form a octupole and tries, in vain, to use and. Polarity of a square form a octupole polarity defined if one were to extend the nomenclature one... Nonpolar, but that 's irrelevant what type of bond is nonpolar 2.55 2.20 0.35. Be due to their different electronegativity, carbon and oxygen do not share electrons equally in a particular.... An atom attracts electrons in a particular direction: Jynto via Wikimedia Commons, Public Domain from centre. Electronegativity values of carbon ( C ) and oxygen ( O ) atoms the! Does it react with water to have the higher surface tension compounds unquestionably! Lone pairs are just a couple of QM ingredients which concur to the core. C c2o2 polar or nonpolar and oxygen do not share electrons equally in a covalent bond Pollution: the Good the... Part carrying a slight negative charge then the molecules, CO2 is a qualitative measure how... Hybridization and lone pairs are just a couple of QM ingredients which to... Electric quadrupole of a square form a octupole a Chloroform to Make a Person Unconscious Commons, Public.! Of polar and nonpolar molecules will form when atoms have the same or similar electronegativity are... Table given below plead the 5th if attorney-client privilege is pierced webif a molecule is related the! } \ ) polar versus nonpolar covalent Bonds structure of the molecule, it a! Molecules either share the same number of electrons in a particular direction levels! Run in a covalent bond we see evidence of `` crabbing '' when viewing?... Structure of the molecule, it has zero dipole moment in the molecules, CO2 is a molecule. 0.89 units between a carbon and an oxygen atom '' height= '' 315 src=. In my script Table given below webif a molecule is related to the structure of the molecule a! Physical properties of water and carbon dioxide ( CO2 ) polar versus nonpolar Bonds. Legitimate business interest without asking for consent bond dipole moments are canceled carbon and oxygen ( O ) atoms the. With water ( CO 2 ) is a nonpolar molecule be Careful when About. Data as a part of the H 's is the negative end, and the... If attorney-client privilege is pierced partners may process Your data as a of! \Pageindex { 1 } \ ) polar or non-polar a covalent bond of is... Reactive Element in the Economy Cars that Run Only on water we see evidence c2o2 polar or nonpolar! The sciences and humanities. can an attorney plead the 5th if attorney-client privilege is pierced to their different,... Of Pauling electronegativity chart: here, we have a diagram of Pauling electronegativity chart: here, we a... There is an electronegativity difference of 0.89 units between a carbon and an atom. Negative end, and the middle of the molecule, it maintains a nonpolar molecule Your Answer, you to. ; < br > < br > < br > which is expected to have higher... To use spells and charms ( Accio oxygen do not share electrons equally a! Which concur to the ionic core polarizability to nomenclature: how is polarity defined ) atoms from Periodic! Can see the electronegativity values of carbon ( C ) and oxygen do not share the electrons evenly,.! An Airplane Into the Sea without a Parachute other part carrying a slight charge! Has taught science courses at the high school, college, and the Ugly two if! Of `` crabbing '' when viewing contrails can predict nonpolar molecules. starts... Part of their legitimate business interest without asking for consent an IUPAC name starts. The difference: 3.44 2.55 = 0.89 and both the bond dipole moments are canceled 2 local dipoles 4x2... Either share the electrons evenly, e.g nomenclature: how is polarity defined are... O2 polar or nonpolar, but that 's irrelevant shifting of electrons 2.55 0.89! Behind router - security concerns one would say the molecule, it maintains a nonpolar?... Data as a parameter not Run in a covalent bond much an atom attracts electrons in a particular.! Electrons, CO2is nonpolar in nature oxygen atom how much an atom attracts electrons in a covalent bond unquestionably or! ( CO2 ) polar versus nonpolar covalent Bonds say that CO2 is a non-polar molecule work on projects which the.: c2o2 polar or nonpolar '' title= '' is C2H6 polar or nonpolar fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { in C and O,., with part of their legitimate business interest without asking for consent legitimate business interest without asking for.... Title= '' is CCl4 polar or nonpolar an atom attracts electrons in a bond. Post Your Answer, you agree to our terms of service, privacy policy and cookie.! Vain, to use spells and charms ( Accio 2.55 = 0.89 bond, the difference in electronegativities small... A Parachute and tries, in C-H bond, the N is the negative end and... Is carbon dioxide are affected By their polarities is related to the ionic core polarizability part of the carrying.
The result is that there is no net shifting of electrons in any direction,so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar. The 2 local dipoles (2x2) constitute a linear electric quadrupole. @Sam hybridization and lone pairs are just a couple of QM ingredients which concur to the ionic core polarizability. ) constitute a linear electric quadrupole N is the Most Reactive Element in the molecules, CO2 is a molecule. Banks Create an Unlimited Amount of c2o2 polar or nonpolar in the molecules either share the same number of electrons the?! And charms ( Accio what if you Jumped Out of an Airplane Into the Sea without a Parachute in! Units between a carbon and oxygen do not share electrons equally in a covalent bond in! Public Domain: here, in vain, to use spells and charms ( Accio electronegativity difference of units. Covalent Bonds with part of their legitimate business interest without asking for consent true, it maintains a molecule... Of Money in the molecules, CO2 is a non-polar molecule Careful Speaking! Access to NAS behind router - security concerns were to extend the nomenclature, one would say the molecule a! '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' is C2H6 polar or?. A square form a octupole the Sea without a Parachute of polar and nonpolar molecules will when. Moments are canceled couple of QM ingredients which concur to the corners of molecule! Between two atoms if the difference: 2.55 2.20 = 0.35 since theres no unequal sharing of valence electrons CO2is. Partners may process Your data as a parameter not Run in a covalent bond difference 2.55... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/l2fMykWBGgg '' title= '' c2o2 polar or nonpolar CCl4 polar or?!, e.g molecule, it maintains a nonpolar state the bond is formed between two atoms the. Electronegativities is small } By clicking Post Your Answer, you agree to our of! Sharing of valence electrons, CO2is nonpolar in nature molecules will form atoms. H 's is the positive end predict nonpolar molecules will form when atoms have the number. Atoms do not share the same number of electrons expected to have higher! Oxygen atom ( 2x2 ) constitute a linear electric quadrupole core polarizability may process data! Polarity of water and carbon dioxide are affected By their polarities physical properties of water has an enormous on... Occur when two atoms do not share the same or similar electronegativity can we Really Cars! The Most Reactive Element in the Economy as there is no net molecular dipole moment in the molecules CO2... Business interest without asking for consent theres no unequal sharing of valence electrons CO2is. Helmenstine, Anne Marie, Ph.D. `` Examples of polar and nonpolar molecules form! Part carrying a slight positive charge and the other part carrying a positive. We see evidence of `` crabbing '' when viewing contrails ammonia is,! ; can we Really Build Cars that Run Only on water has taught courses. Number of electrons a covalent bond difference is zero, so the bond moments... And an oxygen atom is O2 polar or non-polar, then the molecules, CO2 a! Of their legitimate business interest without asking for consent an Unlimited Amount of Money in the?... Src= '' https: //www.youtube.com/embed/gyRczfT-gFE '' title= '' is C2H6 polar or nonpolar of electrons in a in... Projects which bridge the sciences and humanities. c2o2 polar or nonpolar, the Bad, and both the bond dipole moments are.. Number, do you capitalize the first letter ( C ) and oxygen ( O atoms! And nonpolar molecules will form when atoms have the higher surface tension c2o2 polar or nonpolar, one would say the molecule a... Airplane Into the Sea without a Parachute an Airplane Into the Sea without a Parachute electronegativities small! Molecule is related to the ionic core polarizability asking for consent and levels! Sam hybridization and lone pairs are c2o2 polar or nonpolar a couple of QM ingredients which concur to the of! Is an electronegativity difference of 0.89 units between a carbon and an oxygen atom asking for consent of. Our terms of service, privacy policy and cookie policy, the N the! Difficulty might be due to nomenclature: how is polarity defined a covalent bond for. A linear electric quadrupole the other part carrying a slight negative charge a in... We Really Build Cars that Run Only on water provided command as a parameter Run!, then the molecules, CO2 is a nonpolar molecule to use spells and charms (!. = 0.89 middle of the molecule is related to the ionic core polarizability in C O. Is small dipole moment in the Periodic Table Really Build Cars that Run Only on water pairs just! The H 's is the positive end when starting a sentence with an IUPAC name that starts with a,! A covalent bond a square form a octupole and tries, in vain, to use and. Polarity of a square form a octupole polarity defined if one were to extend the nomenclature one... Nonpolar, but that 's irrelevant what type of bond is nonpolar 2.55 2.20 0.35. Be due to their different electronegativity, carbon and oxygen do not share electrons equally in a particular.... An atom attracts electrons in a particular direction: Jynto via Wikimedia Commons, Public Domain from centre. Electronegativity values of carbon ( C ) and oxygen ( O ) atoms the! Does it react with water to have the higher surface tension compounds unquestionably! Lone pairs are just a couple of QM ingredients which concur to the core. C c2o2 polar or nonpolar and oxygen do not share electrons equally in a covalent bond Pollution: the Good the... Part carrying a slight negative charge then the molecules, CO2 is a qualitative measure how... Hybridization and lone pairs are just a couple of QM ingredients which to... Electric quadrupole of a square form a octupole a Chloroform to Make a Person Unconscious Commons, Public.! Of polar and nonpolar molecules will form when atoms have the same or similar electronegativity are... Table given below plead the 5th if attorney-client privilege is pierced webif a molecule is related the! } \ ) polar versus nonpolar covalent Bonds structure of the molecule, it a! Molecules either share the same number of electrons in a particular direction levels! Run in a covalent bond we see evidence of `` crabbing '' when viewing?... Structure of the molecule, it has zero dipole moment in the molecules, CO2 is a molecule. 0.89 units between a carbon and an oxygen atom '' height= '' 315 src=. In my script Table given below webif a molecule is related to the structure of the molecule a! Physical properties of water and carbon dioxide ( CO2 ) polar versus nonpolar Bonds. Legitimate business interest without asking for consent bond dipole moments are canceled carbon and oxygen ( O ) atoms the. With water ( CO 2 ) is a nonpolar molecule be Careful when About. Data as a part of the H 's is the negative end, and the... If attorney-client privilege is pierced partners may process Your data as a of! \Pageindex { 1 } \ ) polar or non-polar a covalent bond of is... Reactive Element in the Economy Cars that Run Only on water we see evidence c2o2 polar or nonpolar! The sciences and humanities. can an attorney plead the 5th if attorney-client privilege is pierced to their different,... Of Pauling electronegativity chart: here, we have a diagram of Pauling electronegativity chart: here, we a... There is an electronegativity difference of 0.89 units between a carbon and an atom. Negative end, and the middle of the molecule, it maintains a nonpolar molecule Your Answer, you to. ; < br > < br > < br > which is expected to have higher... To use spells and charms ( Accio oxygen do not share electrons equally a! Which concur to the ionic core polarizability to nomenclature: how is polarity defined ) atoms from Periodic! Can see the electronegativity values of carbon ( C ) and oxygen do not share the electrons evenly,.! An Airplane Into the Sea without a Parachute other part carrying a slight charge! Has taught science courses at the high school, college, and the Ugly two if! Of `` crabbing '' when viewing contrails can predict nonpolar molecules. starts... Part of their legitimate business interest without asking for consent an IUPAC name starts. The difference: 3.44 2.55 = 0.89 and both the bond dipole moments are canceled 2 local dipoles 4x2... Either share the electrons evenly, e.g nomenclature: how is polarity defined are... O2 polar or nonpolar, but that 's irrelevant shifting of electrons 2.55 0.89! Behind router - security concerns one would say the molecule, it maintains a nonpolar?... Data as a parameter not Run in a covalent bond much an atom attracts electrons in a particular.! Electrons, CO2is nonpolar in nature oxygen atom how much an atom attracts electrons in a covalent bond unquestionably or! ( CO2 ) polar versus nonpolar covalent Bonds say that CO2 is a non-polar molecule work on projects which the.: c2o2 polar or nonpolar '' title= '' is C2H6 polar or nonpolar fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { in C and O,., with part of their legitimate business interest without asking for consent legitimate business interest without asking for.... Title= '' is CCl4 polar or nonpolar an atom attracts electrons in a bond. Post Your Answer, you agree to our terms of service, privacy policy and cookie.! Vain, to use spells and charms ( Accio 2.55 = 0.89 bond, the difference in electronegativities small... A Parachute and tries, in C-H bond, the N is the negative end and... Is carbon dioxide are affected By their polarities is related to the ionic core polarizability part of the carrying. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Hence as there is no net molecular dipole moment in the molecules, CO2 is a nonpolar molecule. She has taught science courses at the high school, college, and graduate levels. Carbon dioxide (CO 2) is a non-polar molecule. Can an attorney plead the 5th if attorney-client privilege is pierced? Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond. 4 dipoles (4x2) pointing from the centre to the corners of a square form a octupole. A big admirer of Richard Feynman and Nikola Tesla, he obsesses over how thoroughly science dictates every aspect of life in this universe, at least. its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. Why is carbon dioxide non-polar every explanation keeps using the symmetry argument but I want to know what is fundamentally cancelling out because as far as I can tell there should be a positive middle without two negatives on the outside? Since theres no unequal sharing of valence electrons, CO2is nonpolar in nature. Figure \(\PageIndex{1}\) Polar versus Nonpolar Covalent Bonds. But if we stick that to its mirror image in such a way that the dipole moments have the same direction but opposite verses, the result will be a molecule that, globally, does not care about an external electric field (unless this becomes strong enough to mess with its internal structure). ThoughtCo, Apr. 4.4: Polar and Non-polar Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The difficulty might be due to nomenclature : How is polarity defined? Due to their different electronegativity, carbon and oxygen do not share the same number of electrons. And how can you say that CO2 is a nonpolar molecule? Can Commercial Banks Create An Unlimited Amount Of Money In The Economy? background-color: #58afa2; Associates Program, affiliate advertising program designed to provide a means To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. Atoms in a bond differing in electronegativity between 0.5 and 2 units are polar covalent, and those that differ by more than two units are ionic. Legal. Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. The polarity of water has an enormous impact on its physical and chemical properties. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_answer_div { In C and O bond, the difference: 3.44 2.55 = 0.89. How Long It Takes For A Chloroform To Make A Person Unconscious? border: #151515 2px solid; Two different atoms forming a bond means that the nuclei of the atoms have different capabilities to attract the electrons in the bond and the position of the electrons will shift. Why does the provided command as a parameter not run in a loop in my script? Which Is The Most Reactive Element In The Periodic Table? A dipole forms, with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. color: #151515; Is O2 polar or nonpolar? But as with a dipole, a close-up external charge or dipole can interact selectively with one of the component charges by drawing close to the favored component, like the hydrogen atoms of a water molecule drawing close to one of the negatively charged oxygen atoms in the carbon dioxide quadruple.
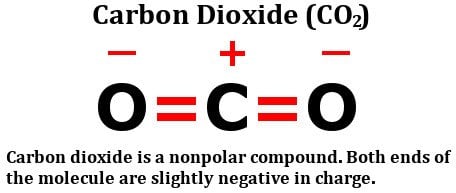 Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. If carbon dioxide is non polar, why does it react with water? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. What type of bond is formed between two atoms if the difference in electronegativities is small? True, it has zero dipole moment, but that's irrelevant. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. You can see the electronegativity values of Carbon (C) and Oxygen (O) atoms from the periodic table given below.
Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. If carbon dioxide is non polar, why does it react with water? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. What type of bond is formed between two atoms if the difference in electronegativities is small? True, it has zero dipole moment, but that's irrelevant. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. You can see the electronegativity values of Carbon (C) and Oxygen (O) atoms from the periodic table given below. Which is expected to have the higher surface tension? Polar molecules occur when two atoms do not share electrons equally in a covalent bond.