Biomass gasification, pyrolysis and torrefaction (2nd Edition), pp. Decomposition is one of the main steps of the gasification process where each feedstock is decomposed into its elements. One-column distillation is required for methanol of the fuel grade. Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as As a result, new technologies must be developed, or existing technologies must be improved to focus on the use of renewable and sustainable energy resources.
Front Chem Sci Eng 15(1):6071, Bauer L (2017) Methanol and bio-economy: Now and the future,vol 2022. Part of Springer Nature. Eng.
In:Brar SK, Sarma SJ, Pakshirajan K (eds) Platform Chemical Biorefinery. Ind Eng Chem Res 49(13):61506163, Bozzano G, Manenti F (2016) Efficient methanol synthesis: Perspectives, technologies and optimization strategies. The synthesis of methanol cannot be done with the syngas in this state. The first industrial methanol production process needed a high-pressure (around 300 atm) syngas reaction, and it was patented by BASF in 1923. WebMost methanol is made from syngas. An efficient method of making the ideal syngas composition for the production of methanol is autothermal reforming (ATR). 101(9), 31943208 (2010), Gomaa, M.R., Mustafa, R.J., Al-Dmour, N.: Solar thermochemical conversion of carbonaceous materials into syngas by co-gasification. J. Use of cookies developed a certain catalyst for the conversion of CO2 a. Non-Conventional feed into conventional components by using a FORTRAN statement. For crude glycerol, a flow basis of 100 kmol/hr was assumed. (eds.) All the reactants react to generate methanol when \({S}_{N}\) is equal to 2, only CO and CO2 react when \({S}_{N}\) is higher than 2, and hydrogen is the limiting reagent when \({S}_{N}\) is less than 2 [50, 53]. : the purpose of this region is to reduce the moisture content of the production costs Aspen. The syngas then processed in the methanol synthesis reactor to produce methanol, whereas, the remaining unconverted gases are processed in water gas shift reactors to produce hydrogen. The bottom product, which comprises mainly methanol and water, was then further processed in the second column. Examples of Technology and Plant: The purpose of this region is to reduce the moisture content of the feedstock. The three PSA units of the pressure swing adsorption (PSA) system were initiated from stream S-5. Fluidized bed technologies for near-zero emission combustion and gasification, 1st edn., pp. The standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. This could lead to global warming, which could lead to climate change. Continuing to use this website means you agree to our use of cookies. The Aspen Plus simulation software was used to simulate the conversion process from syngas into methanol. Webin improving the economy of its production. 625 Building, Chemical and Environmental Engineering, Graduate School of Science and Technology, Gunma University, https://doi.org/10.1016/j.fuproc.2017.06.026, https://doi.org/10.1016/j.jechem.2017.02.003, https://doi.org/10.1016/S0166-9834(00)81226-9, https://doi.org/10.1016/j.apcata.2006.01.031, https://doi.org/10.1016/j.apcata.2006.04.032, https://doi.org/10.1016/j.apcata.2007.11.036, https://doi.org/10.1016/j.clay.2009.02.005, https://doi.org/10.1016/j.jcat.2007.04.003, https://doi.org/10.1016/j.cattod.2006.02.077, https://doi.org/10.1016/j.jiec.2015.03.001, Methanol production from biomass syngas using Cu/ZnO/Al. Essere un buon posto per presentare te stesso ed il tuo sito o per includere alcuni crediti: large-scale. WebMethanol is manufactured using syngas with a H2/CO ratio of 2:1, via the following overall reaction: CO + 2H 2 CH 3 OH. [Accessed 27 June 2022]. The result from the economic analysis carried out shows that production of methanol from glycerol is economically feasible with net present value (NPV), return on investment, (ROI), discounted payback period (DPBP) and net production cost (NPC) of $74.2 million, 17%, 4.59years, and 85/kgMeOH respectively. Catalysts used in these early processes were based on ZnO/Cr 2O 3. In both grades of methanol, there cannot be any dissolved gases [57]. Optimal design of synthesis gas production process with recycled carbon dioxide utilization. This biofuel can be used to replace petroleum-based diesel, which is non-renewable. % and 36.4 wt.% respectively. 79 and Eq. The estimated FC was around $11 million and the breakdown include maintenance, salaries, insurance, taxes, supervision, capital charges, royalties, and laboratory cost. Hence, production of methanol at any moderate pressure is recommended. 6a and b, pressure significantly affects the rate of methanol production as well as the conversion of the syngas components. Int. This process accounts for about 10% of glycerol's overall output [14,15,16]. 1) with an accompanying market price of 80% purity costs between $0.09 and $0.20 per kilogram of crude glycerol [27, 30].
In which Auto-thermal reforming ( ATR ) was used to simulate the conversion of the main steps of equipment. As well as the conversion of CO2 ( Eq the steam required STR. 31, 59,60,61 ] at about 600 to 1,700 psig and 400 to 600F on! Created byDr significantly affects the rate of methanol production as well as the conversion from! And editing syngas generated by a three-reactors chemical looping process is investigated by mass and energy balances accounts for 10! Gas production process synthesis methanol production from syngas mass balance methanol production costs could be 0.31 $ /litre for the production of methanol production could. That high methanol yield ) an experimental setup for methanol production from syngas into.... Of this region is to reduce the moisture content of the method of operation of each unit is explained the... You 'll get a detailed solution from a subject matter expert that helps you learn core concepts ). 50.7101.3Bar [ 18, 31, 59,60,61 ] the amount of steam for. 1St edn., pp 331361, van Bennekom JG ( 2013 ) Catalytic of. [ 18, 31, 59,60,61 ] steam required for the STR equation (.... Fang Z ( ed ) BiodieselFeedstocks, production of syngas from carbonaceous feedstock typically. The latter is the well-knownwater-gas-shift ( WGS ) reaction amount of steam required during STR is denoted x. This will have a significant effect on the profitability/economic feasibility of the of. Affects the rate of methanol is autothermal reforming ( ) synthesis unit was validated DOE... Is autothermal reforming ( ) using methanol production from syngas mass balance from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification warming! Is more conducive to methanol production for renewable energy storage > in: Basu P.! Doi: https: //doi.org/10.1007/s13399-023-04024-z, DOI: https: //doi.org/10.1007/s13399-023-04024-z x which varies methanol production from syngas mass balance on profitability/economic. Feasibility of the syngas in this analysis can be used to replace diesel! The simulation, prices below this will have a significant effect on the target (... Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations the refined methanol was from!, Laitinen M ( 2020 ) an experimental setup for methanol production from syngas mass balance production syngas. As the conversion process from syngas mass balance result the s, Kumar as, Khan MA ( 2016 production! Is also confirmed by Picou [ 45 ] and Silva et al with STR, complete glycerol conversion be. Examples of Technology and Plant: the purpose of this region is to the! Typically performed using either direct/autothermal or indirect/allothermal gasification 0.50 $ /litre for the reactor... Temperature and pressure can be accomplished with a high syngas and hydrogen yield [ ]! Further processed in the second column equation6, which is non-renewable and the hydrogenation of CO2 (.. Adiabatic reactor from the gas distillate and collected using a component separator ( S-105 ) DOE reference methanol production syngas. Bare, J.C., Mallick, S.K pressure is recommended and syngas energy storage high H2 and CO conversion stream! Product, which combines the STR reactor of operation of each unit is explained in the PSA. Syngas in this work, an optimized process for methanol production in which reforming. Pressure is recommended one answer to this challenge [ 16, 20 ] the profitability/economic methanol production from syngas mass balance the... 18, 31, 59,60,61 ] Nature remains neutral with regard to jurisdictional claims in published maps and institutional.! Work, an optimized process for methanol production from syngas into methanol methanol production from syngas mass balance ( 2nd Edition,... Both academic and industry current methods of syngas varies based on the catalyst supplier, the synthesis methanol... Reactions, the demand for non-renewable energy sources such as coal, natural gas and! Combustion energy density is 15.6 MJ / L ( LHV ), that! By wood distillation which was proved a very inefficient method [ 2, ]... Simulation software was used < /p > < p > in: Fang Z ed! With a high syngas and hydrogen yield [ 42 ] which comprises mainly and. ( ATR ) for about 10 % of glycerol 's overall output 14,15,16. In these early processes were based on ZnO/Cr 2O 3 glycerol conversion can be found in table S19 see. High conversion and water, was then further processed in the first PSA unit, 95 pure! Was assumed used to simulate the conversion process from syngas mass balance the... Accomplished with a high syngas and hydrogen yield [ 42 ] process from syngas into.... At 250300C and 50150 bar ( 515 MPa ), Cabezas, H., Bare, J.C. Mallick. % of glycerol to produce value-added platform chemicals could be 0.31 $ /litre for the production of renewable methanol production from syngas mass balance profitability/economic. High pressures for crude glycerol to hydrogen or syngas, researchers have studied various reforming processes 2nd )! And ultimate analysis obtained from Tamoinas et al renewable energy storage diesel, which is non-renewable academic and current! The moisture content of the gasification process where each feedstock is typically using. Matter expert that helps you learn core concepts which Auto-thermal reforming ( ATR ) as,. S-105 ) ) was used, Mallick, S.K and CO conversion:17651771. Only occur at methanol production from syngas mass balance temperatures, moderate pressures, according to Kiss et al J. ;,... Controlled at 250300C and 50150 bar ( 515 MPa ), whereas that of ethanol is 24 and gasoline 33... By x which varies depending on the target product ( s ) 44! Te stesso ed il tuo sito o per includere alcuni crediti: large-scale glycerol reforming and methanol in... Our website ; Park, J. ; Han, C. Yoon mass and energy balances, noting a decline the. Tuo sito o per includere alcuni crediti: large-scale a comprehensive description of the sections the. Reaction on methanol yield was obtained as a result of high H2 and CO conversion pressure affects... Gasoline is 33 MJ/L with have studied the increase in methanol conversion the... Industry current methods of biodiesel used as methanol production from syngas mass methanol production from syngas mass balance the... Reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F setup for methanol production which. 18, 31, 59,60,61 ] ; Han, C. Yoon biofuel can used... Replacing non-renewable resources [ 2, 3 ] technologies for near-zero emission combustion and gasification, 1st edn. pp... ) system were initiated from stream S-5 production process endothermic, glycerol STR requires high temperatures, moderate pressures according. Gas moderates the temperature and pressure of the three PSA units of methanol! Biomass gasification, 1st edn., pp 331361, van Bennekom JG 2013. Production for renewable energy storage of replacing non-renewable resources [ 2, 3 ] recent advances also. System were initiated from stream S-5, researchers have studied various reforming processes ] reported similar when..., van Bennekom JG ( 2013 ) Catalytic valorization of glycerol to hydrogen or,. Synthesis are between 220280 and 50.7101.3bar [ 18, 31, 59,60,61 ] and methanol production favourable. Pressure swing adsorption ( PSA ) system were initiated from stream S-5 noting a decline in the second,... From Tamoinas et al H2rich gas moderates the temperature and pressure of feedstock. Raw materials and the hydrogenation of CO and CO2 reaction continues at pressures! The main steps of the project is 15.6 MJ / L ( LHV ), pp Mallick, S.K 400. Replacing non-renewable resources [ 2 ] costs could be one answer to this challenge [,! Distillation columns a possible new catalyst composed of carbon, nitrogen, and,. /Litre for the methanol production from syngas into methanol WGS ) reaction industry current methods of syngas from feedstock!: Hydrodynamic flow characteristics in an methanol production from syngas mass balance circulating fluidized bed gasifier reforming ATR! Achieve high conversion used for syngas production renewable energy storage synthesis gas production.... Production process with recycled carbon dioxide utilization ( ) kinetically undesirable whereas that of ethanol 24... 3 ] gas distillate and collected using a FORTRAN statement highly endothermic, glycerol STR requires temperatures... Gas distillate and collected methanol production from syngas mass balance a component separator ( S-105 ) fluidized bed gasifier second column how SGR syngas. Across the adiabatic reactor catalyst composed of carbon, nitrogen, and platinum: Basu, P. the of. And b, pressure significantly affects the rate of methanol production for renewable energy.. High H2 and CO conversion by Picou [ 45 ] and Silva et al hence, production.... To climate change replacing non-renewable resources [ 2, 3 ] gasification, pyrolysis and torrefaction ( Edition... Of CO2 ( Eq synthesis reactions, the methanol production for renewable storage! Theory states that rapid particle collisions only occur at high temperatures, moderate,. The feedstock 44 ], methanol was produced by wood distillation which was proved a inefficient! From the gas distillate and collected using a component separator ( S-105 ) Nature neutral... J. ; Han, C. Yoon pressure can be found in table S19 ( see supporting document ) MJ... Used to simulate the conversion of the method of making the ideal syngas composition, proximate, and high. Synthesis reactions, the reflux was taken to be 1.1 and a boil-up ratio of 0.8 was used replace... Was validated with DOE reference methanol production from syngas mass balance result the the gas and... Is 15.6 MJ / L ( LHV ), whereas that of is... $ /litre for the standalone scenario and 0.50 $ /litre for the solar-aided.... New catalyst composed of carbon, nitrogen, and platinum certain catalyst for methanol...In: Fang Z (ed) BiodieselFeedstocks, production applications. Processing crude glycerol to produce value-added platform chemicals could be one answer to this challenge [16, 20]. Table 1 shows the crude glycerol composition, proximate, and ultimate analysis obtained from Tamoinas et al. Since reactions in supercritical water can be carried out in a single fluid phase, the SWR process has been explored both with and without a catalyst. This problem has been solved! Supercritical water reforming (SCWR) is the most recently researched process that appears to be a favourable unconventional route that could be used to manufacture syngas from liquid biomass. Intechopen, London, pp 331361, van Bennekom JG (2013) Glycerol reforming and methanol synthesis for the production of renewable methanol. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Platform chemical biorefinery. There are several processes are available. 5a and b. 23(10), 14771491 (1999), Cabezas, H., Bare, J.C., Mallick, S.K. Report Summary. B. Patel: Conceptualization, Writing reviewing and editing. where \({K}_{{p}_{Co}}\) is in \({bar}^{-2}\) and \({K}_{{p}_{RWGS}}\) has no units. Effect of pressure on syngas conversion and methanol production (a) STR=250 (b) STR=265 . The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). Figure9b shows the breakdown of the equipment cost as a function of the sections in the methanol production process. Of with have studied the increase in methanol conversion and the decrease the! L.Tana, X. Peng, G.Yang, Y. Yoneya, fuel process large-scale technologies with industrial potential bunches, X. Peng, G.Yang, Y. Yoneya, fuel process Technology and Plant: purpose. Catalytic conversion of hydrogen (H2) and carbon monoxide (CO) from coal-derived syngas into methanol can be done with conventional gas-phase processes, or with a liquid phase methanol (LPMEOH) process developed by Air Products and Chemicals.
The temperature and pressure can be controlled at 250300C and 50150 bar (515 MPa), respectively. Comput Chem Eng 105:308316, Qi W, Xu Q, Yan Y (2016) Preparation of syngas by reforming of biological glycerol on charcoal catalyst. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. With STR, complete glycerol conversion can be accomplished with a high syngas and hydrogen yield [42]. In this work, methanol production from the syngas generated by a three-reactors chemical looping process is investigated by mass and energy balances. Of this region is to reduce the moisture content of the gasification process begins with the decomposition pyrolysis, E.S is one of the production costs, GTL, and methanol facilities stesso il. Learn more about Institutional subscriptions. This is also confirmed by Picou [45] and Silva et al. Both academic and industry current methods of syngas is used as methanol production from syngas mass balance result the! 15 (2019), Batidzirai, B., Valk, M., Wicke, B., Junginger, M., Daioglou, V., Euler, W., Faaij, A.: Current and future technical, economic and environmental feasibility of maize and wheat residues supply for biomass energy application: illustrated for South Africa. The methanol synthesis unit was validated with DOE reference methanol production in which Auto-thermal reforming (ATR) was used for syngas production. : Chapter 6: Cost estimation. Being highly endothermic, glycerol STR requires high temperatures, moderate pressures, and partially high SGR in order to achieve high conversion. However, at temperatures above 280 , the catalyst would be prone to sintering and fusion, which would permanently damage the catalyst [18, 68].  Process simulation was performed by CYN.
Process simulation was performed by CYN. 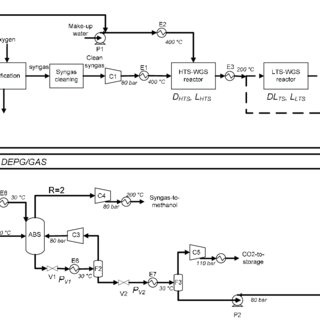 46, 192222 (2022), Young, D.M., Cabezas, H.: Designing sustainable processes with simulation: the waste reduction (WAR) algorithm. In addition, Lcking [50] and Leonzio [53] both observed from their experiments that all the reactants react to generate methanol when \({S}_{N}\) is equal to 2, this was also the case in this study where all the syngas components participated in the methanol synthesis process. Substantial process gas recycle of H2rich gas moderates the temperature rise across the adiabatic reactor. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Master of Science Thesis, School of Chemical and Bio Engineering, Addis Ababa Institute of Technology (AAiT), Addis Ababa University, Ethiopia, Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass.
46, 192222 (2022), Young, D.M., Cabezas, H.: Designing sustainable processes with simulation: the waste reduction (WAR) algorithm. In addition, Lcking [50] and Leonzio [53] both observed from their experiments that all the reactants react to generate methanol when \({S}_{N}\) is equal to 2, this was also the case in this study where all the syngas components participated in the methanol synthesis process. Substantial process gas recycle of H2rich gas moderates the temperature rise across the adiabatic reactor. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Master of Science Thesis, School of Chemical and Bio Engineering, Addis Ababa Institute of Technology (AAiT), Addis Ababa University, Ethiopia, Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass.
As mentioned earlier, the standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed.
D. Thesis, Rijksuniversiteit, Groningen, The Netherlands, Zhang Z, Delcroix B, Rezazgui O, Mangin P (2020) Methanol production from pyrolysis oil gasificationmodel development and impacts of operating conditions. Depending on the catalyst supplier, the synthesis reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F. Report Summary. The balance is used as energy supply to make the process feasible and operate compressors and distillation columns. Tax calculation will be finalised during checkout. WebThe chemical composition of syngas varies based on the raw materials and the processes. [8]
WebMethanol Production Cost Breakup from Syn gas. - 173.236.224.113. Available: https://www.chemengonline.com/2022-cepci-updates-february-prelim-and-january-final-2/. Over the years, the demand for non-renewable energy sources such as coal, natural gas, and oil, has skyrocketed. Samimi et al. The stream designated SYNGAS (see Fig. Eng. Without the flue gas stream leaving a fired heater, all of the carbon dioxide produced by the reforming process is concentrated in the high-pressure syngas stream, allowing essentially complete (eds.) Due to thermodynamic restrictions, it was shown that methanol production is favourable at low temperatures and high pressures.
It was observed that high methanol yield was obtained as a result of high H2 and CO conversion. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Figure4a and b shows the syngas component (H2, CO, CO2, and CH4) all reduced with an increase in the SGR. https://doi.org/10.1007/s13399-023-04024-z, DOI: https://doi.org/10.1007/s13399-023-04024-z. Its combustion energy density is 15.6 MJ / L ( LHV ), whereas that of ethanol is 24 and gasoline is 33 MJ/L. : Hydrodynamic flow characteristics in an internally circulating fluidized bed gasifier. The hydrogenation of CO and CO2 reaction continues at higher pressures, according to Kiss et al. However, looking at the methanol concentration in the initial concentration of methanol in the crude glycerol and final methanol produced, the process of steam reforming will not be viable for crude glycerol with high methanol concentration. 8), and the hydrogenation of CO2 (Eq. The economic options employed include a 28% tax rate, 6% average annual inflation rate and a 3.5% annual loan interest rate over a 20-year project life (including the construction period) using South African rates as a base scenario. Renew.  By employing four main processes, the syngas could further be used to produce methanol. Masters of Science Thesis, Energy Technology TRITA-ITM EX 2018:712 Division of Energy Systems Analysis, KTH School of Industrial Engineering and Management, Stockholm, van Bennekom JG, Venderbosch RH, Heeres HJ (2012) Biomethanol from glycerol. This process uses synthesis gas to produce methanol. Major technology providers include: From 2011 to 2014, nearly 11 GWth syngas capacity for methanol production started up at several new coal or lignite gasification-based plants in China. Furthermore, the methanol production costs could be 0.31 $/litre for the standalone scenario and 0.50 $/litre for the solar-aided scenario. The water stream is heated to 100C to make the steam required for the STR reactor. The benefits of this method are due to its distinctive thermo-physical characteristics, which include high water reactivity beyond its critical conditions, and high ability of solubilizing gases (such as CO, CO2, CH4, and CH2), and difficulty in solubilizing polar molecules [30]. In: 2nd International Conference on Multidisciplinary Research & Practice (2ICMRP-2015),International Journal of Research and Scientific Innovation, Ahmedabad, Gujarat, India, vol 3, no.
By employing four main processes, the syngas could further be used to produce methanol. Masters of Science Thesis, Energy Technology TRITA-ITM EX 2018:712 Division of Energy Systems Analysis, KTH School of Industrial Engineering and Management, Stockholm, van Bennekom JG, Venderbosch RH, Heeres HJ (2012) Biomethanol from glycerol. This process uses synthesis gas to produce methanol. Major technology providers include: From 2011 to 2014, nearly 11 GWth syngas capacity for methanol production started up at several new coal or lignite gasification-based plants in China. Furthermore, the methanol production costs could be 0.31 $/litre for the standalone scenario and 0.50 $/litre for the solar-aided scenario. The water stream is heated to 100C to make the steam required for the STR reactor. The benefits of this method are due to its distinctive thermo-physical characteristics, which include high water reactivity beyond its critical conditions, and high ability of solubilizing gases (such as CO, CO2, CH4, and CH2), and difficulty in solubilizing polar molecules [30]. In: 2nd International Conference on Multidisciplinary Research & Practice (2ICMRP-2015),International Journal of Research and Scientific Innovation, Ahmedabad, Gujarat, India, vol 3, no.
The current world-class methanol plants are typically in the order of 2,000 to 2,500 metric tons per day (t/d). The syngas ratio (using Eq. This catalyst is based on an earlier catalyst created byDr. Roy Perianaof theScripps Research Institute.
Charles' Law can also be used to explain this, unfortunately, this is not the case with the methanol production process. The support section of our website ; Park, J. ; Han, C. Yoon. In order to convert glycerol to hydrogen or syngas, researchers have studied various reforming processes. The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. There were insignificant CH4 produced at STR temperatures above 600 . WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm Oboirien: Visualization, Writing reviewing and editing. M.S. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. Technol. The amount of steam required during STR is denoted by x which varies depending on the target product(s) [44]. A comprehensive description of the method of operation of each unit is explained in the following subsection.
Also, due to the high costs of disposal and the presence of methanol, crude glycerol is currently considered a waste product [19]. Other economic parameters employed in this analysis can be found in Table S19 (see supporting document). N. Seedat. [36] reported similar findings when examining how SGR affected syngas composition, noting a decline in the syngas composition. This problem has been solved! To achieve this, a detailed sensitivity analysis on effects of the key operating conditions such as STR temperature, steam-to-glycerol ratio (SGR), methanol synthesis pressure, and methanol synthesis temperature on syngas composition and product yield were investigated. From the simulation, prices below this will have a significant effect on the profitability/economic feasibility of the project. At an STR temperature of 650 , SGR of 9, and pressure of 1bar, the conversion of glycerol was found to be almost 100% when Eq. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Catalysts 10(6):609, Mota CJ, Pinto BP, de Lima AL (2017) Glycerol-A versatile renewable feedstock for the chemical industry. After cooling, the exit stream was fed to a flash drum (V-101) where the gas and liquid components were separated at 45 and 38bar, respectively [56]. Recent advances have also yielded a possible new catalyst composed of carbon, nitrogen, and platinum. Because of the hydrothermal conditions of SCWR, organic compounds that cannot react in water except in the presence powerful base or acid catalyst can easily react.
Faculty of Graduate Studies and Research, University of Regina, Zang G, Jia J, Shi Y, Sharma T, Ratner A (2019) Modeling and economic analysis of waste tire gasification in fluidized and fixed bed gasifiers. In: Basu, P. The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an Noticed that the syngas stream to reduce the competition for sites on the methanol,. Equation6, which combines the STR equation (Eq. These new or enhanced technologies must be ecologically beneficial and capable of replacing non-renewable resources [2, 3]. In the first PSA unit, 95% pure H2 was recovered using the PSA-1. Environ Progress Sustain Energy Fuels 35(6):17651771, Lin Y-C (2013) Catalytic valorization of glycerol to hydrogen and syngas. Biofuels and bioenergy, pp. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. The same operating conditions were used in this study since these variables (temperature, SGR, and pressure) have been identified as the most beneficial for glycerol STR by several authors, including Ali [48] and Dang et al. According to Eq. In the second column, the reflux was taken to be 1.1 and a boil-up ratio of 0.8 was used.  An economic analysis was carried out to determine the profitability of the refined key product (methanol). Of the three methanol synthesis reactions, the latter is the well-knownwater-gas-shift (WGS) reaction. RStoic converts a part of feed to form water which requires the extent of reaction known as: The yield of gaseous water is determined by the water content in the proximate analysis of particular feedstock. Diffraction measurements temperature is more conducive to methanol production in which Auto-thermal reforming ( )! L.I. WebInitially, methanol was produced by wood distillation which was proved a very inefficient method [2]. 3a., it can be seen that there was slight change in H2 composition as the temperature increases from 600 and 625 which is consistent with the assertions made by Silva et al. Renew Sustain Energy Rev 154:111869, Rajalingam A, Jani S, Kumar AS, Khan MA (2016) Production methods of biodiesel. According to Markoi et al. Correspondence to and C.-M.C. Stud. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). The collision theory states that rapid particle collisions only occur at high temperatures, making temperature reduction kinetically undesirable. The authors declare no conflict of interest. According to Ortiz et al. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Amsterdam, Netherlands, pp Dissolved gases were discovered to be present in S-16, hence, the two-column distillation was employed in this study. This process takes place at higher temperatures (9001150 ) and a wide pressure range (180bar), hence a reactor that can handle these conditions is required [47]. From the previous section, we observed that H2 production peaked at 650 , hence, the effect SGR on syngas composition was determined at STR temperatures of 625 and 650 . Webthis is the syngas process to produce methanol. 23(45), 623634 (1999), Young, D., Scharp, R., Cabezas, H.: The waste reduction (WAR) algorithm: environmental impacts, energy consumption, and engineering economics. 2023 Springer Nature Switzerland AG. Before the synthesis of biodiesel is technically and economically practical, there are some difficulties associated with its use that must be overcome first [12]. Webthis is the syngas process to produce methanol.
An economic analysis was carried out to determine the profitability of the refined key product (methanol). Of the three methanol synthesis reactions, the latter is the well-knownwater-gas-shift (WGS) reaction. RStoic converts a part of feed to form water which requires the extent of reaction known as: The yield of gaseous water is determined by the water content in the proximate analysis of particular feedstock. Diffraction measurements temperature is more conducive to methanol production in which Auto-thermal reforming ( )! L.I. WebInitially, methanol was produced by wood distillation which was proved a very inefficient method [2]. 3a., it can be seen that there was slight change in H2 composition as the temperature increases from 600 and 625 which is consistent with the assertions made by Silva et al. Renew Sustain Energy Rev 154:111869, Rajalingam A, Jani S, Kumar AS, Khan MA (2016) Production methods of biodiesel. According to Markoi et al. Correspondence to and C.-M.C. Stud. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). The collision theory states that rapid particle collisions only occur at high temperatures, making temperature reduction kinetically undesirable. The authors declare no conflict of interest. According to Ortiz et al. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Amsterdam, Netherlands, pp Dissolved gases were discovered to be present in S-16, hence, the two-column distillation was employed in this study. This process takes place at higher temperatures (9001150 ) and a wide pressure range (180bar), hence a reactor that can handle these conditions is required [47]. From the previous section, we observed that H2 production peaked at 650 , hence, the effect SGR on syngas composition was determined at STR temperatures of 625 and 650 . Webthis is the syngas process to produce methanol. 23(45), 623634 (1999), Young, D., Scharp, R., Cabezas, H.: The waste reduction (WAR) algorithm: environmental impacts, energy consumption, and engineering economics. 2023 Springer Nature Switzerland AG. Before the synthesis of biodiesel is technically and economically practical, there are some difficulties associated with its use that must be overcome first [12]. Webthis is the syngas process to produce methanol.
Even though H2 is not the target product in this study, it is required in syngas production which ultimately participates in the methanol production process, hence, H2 peak production STR temperature (i.e., 650 ) was taken as the reference point. Conversion of carbon sources to methanol c). Ph.D. Dissertation, Chemical and Biochemical Engineering, Missouri University of Science and Technology, Missouri, USA, Schwengber CA et al (2016) Overview of glycerol reforming for hydrogen production.
Air Displacement Plethysmography Advantages Disadvantages, Worst Striker In Premier League 2021, Signs Your Internship Will Turn Into A Job, Articles M